TECHNOLOGY

Electro Deionisation (EDI) is a water treatment technology that utilizes electricity, ion exchange membranes, and ion exchange to deionize water. Electro deionisation is a very efficient as well as environmentally friendly process for the treatment of water.
How Does EDI Work?
- Electrolysis
The fundamental process begins with electrolysis. Imagine a simple model of a battery connected to two electrodes immersed in a saltwater bath.
- At the cathode, a reduction reaction occurs: hydrogen gas is released, and OH- ions
- At the anode, an oxidation reaction takes place: oxygen gas is released, and H+ ions
- Salt in the solution facilitates continuous reactions, drawing hydroxyl ions from the cathode and hydrogen ions from the anode.
- Electrodialysis
In electrodialysis, electrical current drives ions across a semipermeable membrane.
- EDI System
In an EDI system:
- A membrane allowing cations (OH- ions)only is positioned next to the cathode.
- A membrane permeable to anions (H+ ions)only is positioned next to the anode.
- A central chamber contains the saline solution.
- When electrical charge is applied, ions move across the membranes to their respective electrodes, leaving behind salt constituents and impurities
Application of EDI
Electro Deionization (EDI) is used to obtain brine water from seawater, the process is adapted to focus on separating and concentrating the salts instead of removing them.
Here’s how it generally works:
- Initial Seawater Intake
The process begins by taking seawater as the source. Seawater is naturally salty because it contains various salts and minerals.
- Electro Deionization (EDI)
In this application, EDI is used to concentrate the salts in the seawater rather than removing them. The electric field is applied to separate and concentrate the ions, making the water more brine-like.
- Ion Separation
The EDI system’s membranes and electrodes help in separating the positive and negative ions in the seawater, concentrating the salts in one part of the water.
- Brine Collection
The concentrated solution, now more like brine due to the increased salt content, is collected. This brine water can be further processed or used for specific industrial applications that require high salt concentrations.
- Purified Water Output
The remaining portion of the water, after the concentration of salts, can be considered as relatively purified water. While not completely desalinated, it may have a lower salt content compared to the initial seawater.
The EDI process is adapted to concentrate salts, resulting in brine water as the output. This brine water will be useful in salt industries. This process focusses on salt production rather than freshwater generation.
Technical Specifications:
- 10-unit EDI Module
- 50m3/hour
- recovery rates (%):75-85
- Resistivity Ω .cm)≥15
- Working voltage VDC)20-50
- Working current(ADC)0
- Working Pressure(MPa)15-0.4
- Maximum pressure(MPa)4
- Frequency:50 Hz
- Input Voltage: 220V AC
- Output Voltage: 0 – 200 VDC
- Output current: 0- 6.5A
- power consumption:≤ 8 W
- Working temperature: 0-45℃
Vacuum Evaporator is a machine that uses heat and a vacuum to take the water out of seawater, leaving the salt behind for collection. It’s like a special tool to make salt from salty water. Europe, Asia, or elsewhere, the use of a Vacuum Evaporator for salt production is a common and effective method used globally to harness salt from seawater.
Here’s how it generally works:
The Vacuum Evaporator plant is designed to treat water-based liquid waste output from the most diverse industrial production lines.
A system that creates a vacuum within a boiler allows the boiling point of the water-based waste liquid to be treated at a temperature inferior to that of normal atmospheric pressure. The temperature so obtained is between 20⁰C and 70⁰C.
This method allows water and other volatile compounds to evaporate, while all other byproducts will condensate within the boiler.
The evaporating water is condensed, by a steam exchanger within the plant, and reaches a liquid state again.
The energy needed to boil the liquid waste is supplied by hot water or a heat pump, while the water used for vapor condensation is supplied by an external refrigeration system, such as a cooling tower, or the previously mentioned heat pump.
The hydraulic vacuum circuit within the boiler is inserted into the evaporator start-up, switching on the liquid-ring vacuum pump and the check valve, which, by a closed circuit and a Venturi tube, generate the hydraulic vacuum within the ebullition chamber and the condensation chamber.
During the evaporator rest periods, for discharge of the final concentrate, depletion of the liquid to be treated, and so on, retention of the vacuum is secured by the check valve.
During the evaporation process, the vacuum is secured by the continuous operation of both the vacuum pump and the opening of the check valve.
Application of Vacuum Evaporator
- Wastewater Treatment
- Vacuum evaporators are used to treat industrial wastewater by concentrating the contaminants and reducing the volume of liquid to be disposed of.
- Salt Production
- Vacuum evaporators play a crucial role in the production of salt by removing water from seawater or brine, leaving behind concentrated salt.
- Chemical Processing
- Industries involved in chemical production utilize vacuum evaporators to concentrate and separate chemical solutions, facilitating the recovery of valuable compounds.
Technical Specifications:
- Electrical voltage (Volt): 220V
- Frequency (Hz): 60
- Noise level at 10 mt (dB): max 80
- Distillate flowrate with tap water supply: +/- 10% lt/h 5
- Installed electric power kW/h: 4
- Electric power consumption with tap water supply: kW/h +/- 10%
- Compressed air pressure (bar): 6
- Compressed air flow (m3/h): 1
- Freon gas: R134A
- Boiling chamber: SAF2507
- Condensation chamber: AISI316L
- Vacuum pump tank: AISI316L
- Heating exchanger: SAF2507
- Cooling exchanger: AISI316L
- Vacuum pump: AISI316L
- Concentrate discharge/recirculation pump Not present.
- Pneumatic valves: PVC-EPDM
- Frame: AISI304
- Supports: AISI304
- Piping: PVC-EPDM
- Vacuum manometer: Analogue with PLC reading
- Boiling chamber levels: Internal floating levels
- Distillate levels: Internal floating levels
- Pressure switches: Danfoss

The Porometer and a Membrane Automatic Casting Machine offers an efficient and precise approach to membrane manufacturing. The Membrane Automatic Casting Machine automates the production process, crafting thin membranes with consistency and accuracy. These membranes play a crucial role in various industries, such as filtration and separation applications.
Here’s how it generally works:
- Membrane Automatic Casting Machine:
- The process begins with the Membrane Automatic Casting Machine. This specialized equipment automates the creation of thin membranes or layers from specific materials.
- The machine precisely casts or forms these membranes, ensuring uniform thickness and quality. This automated process enhances efficiency and consistency in membrane production.
- Membrane Production:
- As the Membrane Automatic Casting Machine operates, it produces membranes with specific characteristics, such as thickness and composition. These membranes are essential components used in various applications, including filtration systems.
Application of Vacuum Evaporator
- Filtration Industry
- One primary application is in the filtration industry. The Membrane Automatic Casting Machine produces membranes with specific characteristics suitable for filtration purposes. The Porometer is then used to ensure that these membranes have the desired pore size and distribution, optimizing their efficiency in separating particles during filtration processes.
- Biomedical and Pharmaceutical Applications
- In biomedical and pharmaceutical fields, the combination is applied to produce membranes used in drug delivery systems, diagnostics, and other medical devices. The Porometer ensures that the membranes meet strict standards for controlled release and selective permeability.
- Water Purification
- The technology is employed in water purification systems where membranes are used to separate impurities from water. The Porometer verifies the quality of these membranes, ensuring they effectively filter out contaminants while allowing the passage of clean water.
Technical Specifications:
- Casting size: Approximately 350 x 560 mm
- Casting speed: 0.1 up to 10 cm/s
- Heating of the coating surface: Up to 60 °C
- Display: Digital display (touch screen) to set temperature and read the actual temperature, as well as to set and read the coating (casting) speed.

Double Beam UV-Visible Spectrophotometer is a scientific instrument used for measuring the absorption of ultraviolet (UV) and visible light by a substance.
Here’s how it generally works:
- Light Source
- The instrument has a light source that typically includes both ultraviolet (UV) and visible light components. Deuterium lamps provide UV light, while tungsten or xenon lamps supply visible light.
- Splitting the Beam
- The incoming light beam is split into two paths: the sample beam and the reference beam. This is a key feature of the double beam design.
- Sample and Reference Measurements
- The sample beam passes through the sample compartment where the substance to be analyzed is placed. Simultaneously, the reference beam travels through a reference material or a blank solution.
- Absorption by Sample
- As the sample beam passes through the sample, the substance in the sample absorbs specific wavelengths of UV or visible light. The amount of absorption is directly related to the concentration of the absorbing species in the sample.
- Reference Measurement
- The reference beam, which has not passed through the sample, provides a baseline measurement. It represents the intensity of light without the influence of the sample.
- Detector Response
- Both the sample and reference beams reach a detector (commonly a photodiode array or photomultiplier tube) that measures the intensity of light at different wavelengths.
- Data Comparison
- The spectrophotometer compares the intensity of the sample beam to that of the reference beam at each wavelength. The resulting data is used to calculate the absorbance of the sample.
- Calculation of Absorbance
- The absorbance (A) is calculated using the formula: A = -log10(I/I₀), where I is the intensity of the sample beam, and I₀ is the intensity of the reference beam.
- Display and Output
- The calculated absorbance values are typically displayed on the instrument’s screen. Users may also have the option to save or export data for further analysis.
Application of Double Beam UV Visible Spectrophotometer
Teaching and Research
- Used in educational institutions for teaching basic principles of spectroscopy and in-depth research projects.
Water Quality Testing
- Monitoring the quality of drinking water and wastewater by analyzing absorption characteristics.
Food and Beverage Industry
- Determining the concentration of additives, colorants, and other components in food and beverages.
Quantitative Analysis
- Determining the concentration of a substance in a solution based on the amount of light absorbed. This is widely used in chemistry, biochemistry, and environmental science.
Qualitative Analysis
- Identifying the presence or absence of specific compounds in a sample by analyzing their absorption spectra.
Biological and Biochemical Research
- Studying biomolecules like proteins, nucleic acids, and enzymes by measuring their absorbance characteristics.
Technical Specifications:
- Wavelength Range: 190-1100 nm
- Wavelength Accuracy: ±0.3 nm
- Wavelength Repeatability: 0.2 nm
- Spectral Bandwidth: 1.8 nm
- Photometric Range: 0-200%T, -0.3-3.0 A
- Photometric Accuracy: ±0.3%T or ±0.003A @ 1A
- Stability: 0.0003 A/h @ 500nm
- Baseline Flatness: ±0.0005 A
- Stray Light: 0.04%T @ 220 nm, 360 nm
- Light Source: Tungsten and Deuterium Lamp
- Software: Optional PC model with UV/Vis analyst software
- Gross Dimension (W/D/H): 589mm x 428mm x 200 mm
- Display: 5 inches LCD (320*240 dots)
- Weight (Net/Gross): 22 kg
- Power Supply: AC 110/220 V 60 Hz

Atomic absorption spectrophotometer is a scientific device used to measure the amount of specific metals in a sample. Imagine you have a solution with some metal in it, like copper or zinc. The spectrophotometer works by shining light through this solution. The metal atoms in the solution absorb some of this light at specific wavelengths.
Here’s How It Generally Works:
- Light Source
The atomic absorption spectrophotometer starts with a light source, usually a hollow cathode lamp, which emits light at specific wavelengths that are characteristic to the metal being analyzed.
- Sample Introduction
Your sample, which contains the metal you want to measure, is turned into a fine mist or vapor. This can be done using techniques like nebulization or flame atomization.
- Absorption of Light
The light from the lamp passes through the sample. If the metal is present, its atoms absorb light at specific wavelengths. This is due to the electrons in the metal atoms jumping to higher energy levels when they absorb this light.
- Detector
The remaining light that passes through the sample reaches a detector. The detector measures how much light was absorbed by the metal atoms in the sample.
- Analysis
The spectrophotometer compares the intensity of the light before and after it passes through the sample. The amount of absorbed light is directly proportional to the concentration of the metal in the sample.
- Quantification
Scientists use this information to quantify the concentration of the metal in the sample. The more intense the absorption, the higher the concentration of the metal.
Application of Atomic Absorption Spectrophotometer
- Environmental Analysis
Scientists use atomic absorption spectrophotometry to analyze water and soil samples for metal contamination. It helps identify pollutants like lead, mercury, and cadmium, contributing to environmental monitoring and protection.
- Clinical and Biological Studies
In the medical field, this tool is used to analyze blood and tissue samples. It helps in diagnosing conditions related to metal imbalances in the body, such as iron deficiency or toxic metal exposure.
- Quality Control in Industries
Industries like pharmaceuticals, food and beverage, and electronics use atomic absorption spectrophotometers to ensure the quality of their products. For example, measuring the concentration of metals in pharmaceuticals or checking the metal content in food products.
- Agricultural Research
Researchers in agriculture use atomic absorption spectrophotometry to analyze soil samples. This helps in understanding the nutrient content and potential metal contaminants in soils, guiding proper crop management.
Technical Specifications:
- Pump
- Parallel-type double plunger.
- Pulsation: ≤ 0.1 MPa at 1.0 mL/min, 10 MPa (Water).
- Flow rate setting range: 0.0001 to 10 mL/min.
- Flow rate accuracy: ≤ ±1% or ≤ ±2 μL/min (specified conditions).
- Autosampler
- Injection volume accuracy: ±1% (50 μL, N = 10).
- Injection volume setting range: 0.1 to 100 μL.
- Injection volume reproducibility: RSD < 0.20% (5.0−2000 μL).
- Column Oven
- Heating and cooling method: Forced air circulation.
- Temperature control range: Room temperature −12 °C to 90 °C.
- UV Detector
- Wavelength range: 190 to 700 nm.
- Wavelength accuracy: ≤ ±1 nm.
- Noise: ≤ ±2.5 × 10⁻⁶ AU (250 nm, specified condition).
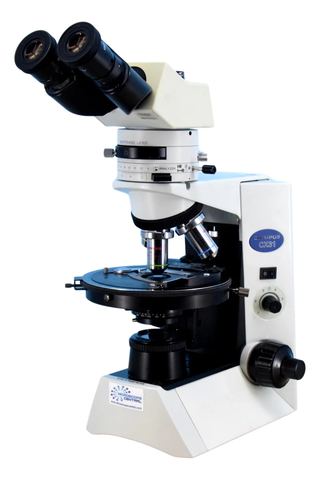
A polarized microscope is a specialized type of microscope used in microscopy, particularly for examining minerals, crystals, and other materials that interact with polarized light.
Here’s How It Generally Works:
An Atomic Absorption Spectrophotometer works by measuring how much light is absorbed by specific atoms in a sample. Here’s a simple step-by-step:
- Light Source
The spectrophotometer uses a light source to emit light at specific wavelengths, usually through a hollow cathode lamp.
- Sample Introduction
The sample, often a liquid containing the element of interest, is turned into a fine mist or vapor.
- Shining Light
The light passes through the mist, and if the element is present in the sample, its atoms will absorb some of the light at specific wavelengths.
- Detector
A detector measures how much light is absorbed by the atoms in the sample.
- Analysis
By comparing the amount of light before and after passing through the sample, the spectrophotometer calculates the concentration of the element in the sample.
Application of Atomic Absorption Spectrophotometer
- Environmental Analysis: To measure metal concentrations in water, soil, and air.
- Clinical Chemistry: Determining elements in biological samples for medical diagnosis.
- Food and Beverage Industry: Analyzing metal content in food products for quality control.
- Geological Studies: Identifying and quantifying elements in rocks and minerals.
- Material Science: Analyzing metal content in various materials.

